Hsp90 chaperone proteins play a key role in stabilizing cancer-driving proteins. But pan-Hsp90 inhibitors have failed in clinical development due to on-target toxicity, especially in the heart and eyes, and disappointing efficacy results.

For decades, the scientific community has recognized the immense potential of Hsp90 inhibition. Our platform is built on the selective inhibition of Hsp90β, one of several isoforms within a validated cancer target.
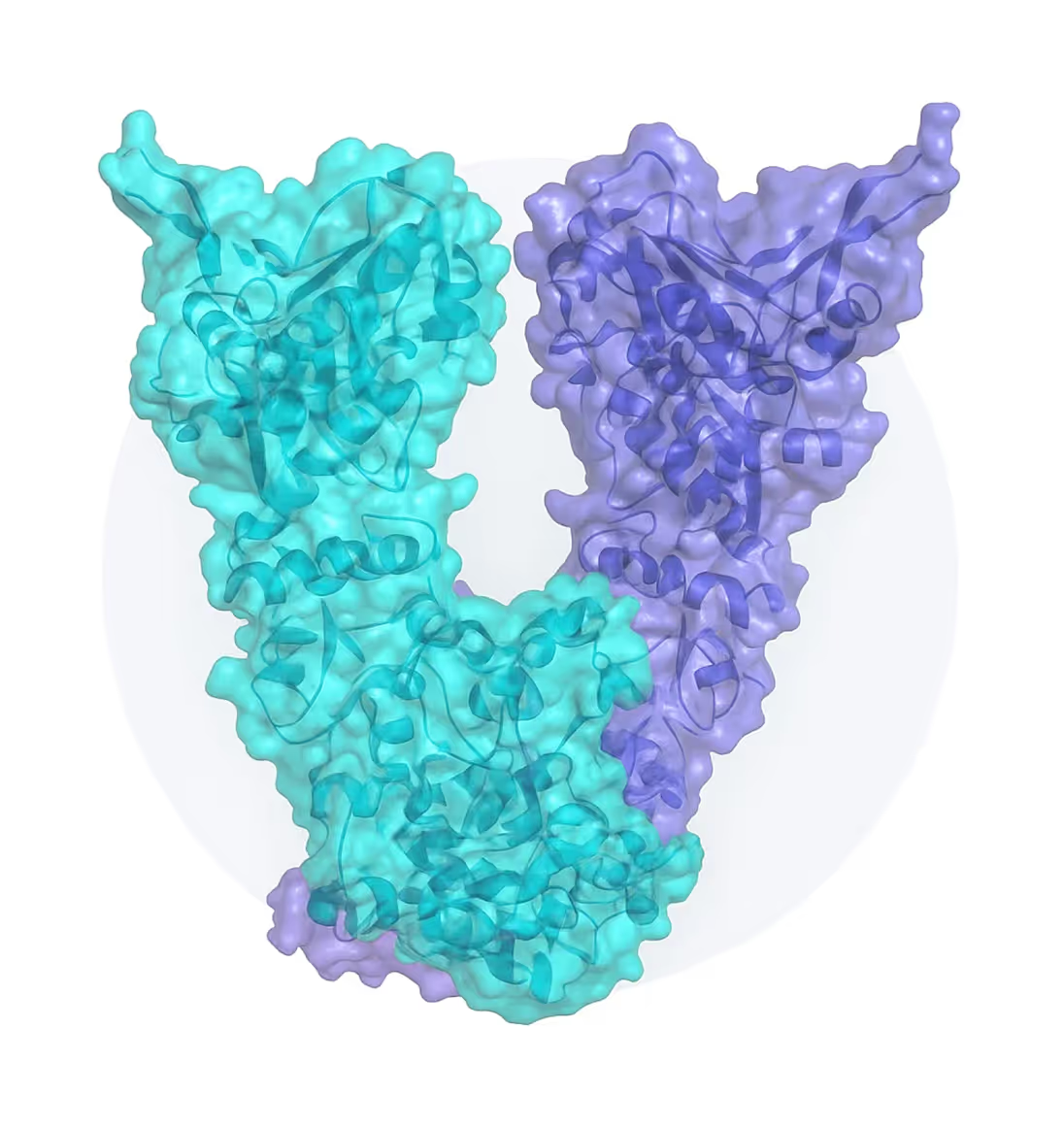
While other therapies inhibit multiple Hsp90 isoforms, Grannus is developing a first-in-class inhibitor that targets Hsp90β exclusively, delivering potent anti-tumor activity with a clean safety profile finally unlocking the full promise of this proven cancer target.
Hsp90 chaperone proteins play a key role in stabilizing cancer-driving proteins. But pan-Hsp90 inhibitors have failed in clinical development due to on-target toxicity, especially in the heart and eyes, and disappointing efficacy results.

Ovarian cancer has emerged as a leading indication due to its high unmet need and encouraging preclinical data. This innovative approach holds substantial promise for expansion into other challenging cancer types, including prostate, triple-negative breast cancer (TNBC), and pancreatic cancer, representing significant opportunities for future clinical applications.
At Grannus, we've learned from these lessons and refined the approach. Our Hsp90β-selective inhibitor avoids these toxicities while preserving tumor-killing power.
> 100 fold selective to Hsp90β vs other isoforms
> 50% oral bioavailability with preferential tumor biodistribution
Oral in vivo POC in overian cancer, and additional data in other tumors
What makes our approach different is selectively inhibiting the beta isoform, creating a clean toxicity profile
Issued US patent protection through 2039, with provisional filings extending protection to 2045
With deep expertise in drug development, oncology, and translational science, the Grannus team isn't just following a path; we're forging a new one. We’re advancing GRE317 with IND-enabling studies, in vitro ADME, PK/PD profiling, and biomarker discovery to enable precision oncology trials.